vol. 27, No. 8, 2016, p. 1319-1519
5th BCNP and XXXI RESEM: a Tribute to Biodiversity
Dreamers make dreams come true said Fed Kavli a Norwegian who wanted to help scientists to make the world a better place for future generations (1927‑2013). His dreams relied on astrophysics, nanoscience and neuroscience because he believed these were the tools for the future. However in my dreams I have always thought that natural products were important because they can lead us anywhere in Science. They were the origin of our life and will be our end if we do not take care of them.
(Read more at Editorial)
Special Issue on the 5th Brazilian Conference on Natural Products and XXXI Meeting on Micromolecular Evolution, Systematics and Ecology
Editorial J. Braz. Chem. Soc. 2016, 27(8), 1319
Review J. Braz. Chem. Soc. 2016, 27(8), 1320-1333
Natural Product-Derived Drugs Based on β-Adrenergic Agents and Nucleosides
David J. Newman
How to cite this article
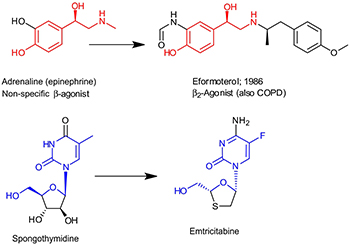
The influence of natural products on β-agonists/antagonists, anti-tumor and antiviral agents.
https://dx.doi.org/10.5935/0103-5053.20160070
J. Braz. Chem. Soc. 2016, 27(8), 1334-1338
Turning Metabolomics into Drug Discovery
Asmaa Boufridi; Ronald J. Quinn
How to cite this article
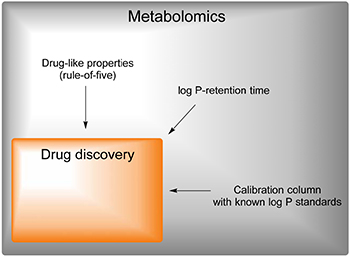
Limit metabolome space to drug space. Drug-like properties - Lipinski's rule-of-five. Use standards with known physicochemical properties to calibrate the column. Correlate log P to retention time (tR).
https://dx.doi.org/10.5935/0103-5053.20160083
J. Braz. Chem. Soc. 2016, 27(8), 1339-1345
Developing Commercial Production of Semi-Synthetic Artemisinin, and of β-Farnesene, an Isoprenoid Produced by Fermentation of Brazilian Sugar
Kirsten R. Benjamin; Iris R. Silva; Joao P. Cherubim; Derek McPhee; Chris J. Paddon
How to cite this article
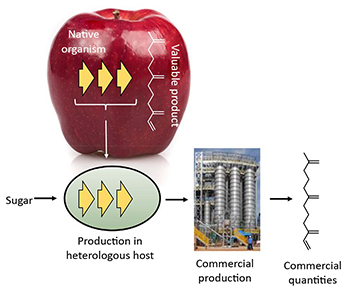
Genes encoding the biosynthetic pathway for production of a valuable product (e.g., farnesene) in a native organism are expressed in a heterologous microbial host (e.g., yeast). The engineered yeast produces farnesene by commercial fermentation. Copyright c 2016 Amyris, inc. All rights reserved.
https://dx.doi.org/10.5935/0103-5053.20160119
J. Braz. Chem. Soc. 2016, 27(8), 1346-1354
Elucidating the Mode of Action of Marine Natural Products through an Immunoaffinity Fluorescent (IAF) Approach
James J. La Clair; William Fenical; Leticia V. Costa-Lotufo
How to cite this article
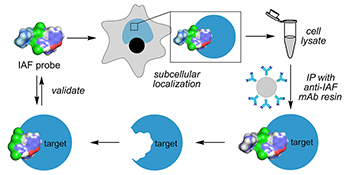
Elucidating the mode of action (MOA) and associated biomolecular targets of a natural product is often one of the bottlenecks in the drug discovery process. We present an overview of recent developments on an immunoaffinity fluorescent (IAF) approach that unites cellular microscopy with immunoaffinity target identification to rapidly characterize and subsequently validate the targets and cellular activity of natural products.
https://dx.doi.org/10.5935/0103-5053.20160148
J. Braz. Chem. Soc. 2016, 27(8), 1355-1378
The Genus Psychotria: Phytochemistry, Chemotaxonomy, Ethnopharmacology and Biological Properties
Nivea O. Calixto; Meri Emili F. Pinto; Suelem D. Ramalho; Marcela C. M. Burger; Antonio F. Bobey; Maria Claudia Marx Young; Vanderlan S. Bolzani; Angelo C. Pinto
How to cite this article
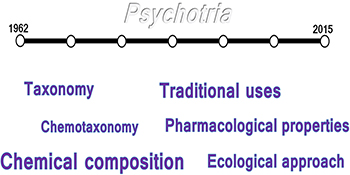
Highlighting the relevant literature from 1962 until 2015, on taxonomy, chemotaxonomy, traditional uses, pharmacological properties, chemical composition and ecological approach from Psychotria genus.
https://dx.doi.org/10.5935/0103-5053.20160149
J. Braz. Chem. Soc. 2016, 27(8), 1379-1397
Benzoxazinoids: Reactivity and Modes of Action of a Versatile Class of Plant Chemical Defenses
Felipe C. Wouters; Jonathan Gershenzon; Daniel G. Vassao
How to cite this article
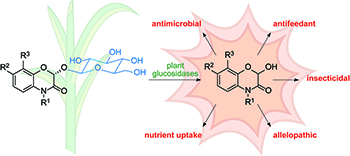
Benzoxazinoids constitute a diverse family of plant secondary metabolites with defensive roles against herbivores, pathogens, and competing plants. Their chemical properties and possible modes of action are discussed in the context of their biological activities.
https://dx.doi.org/10.5935/0103-5053.20160177
Articles J. Braz. Chem. Soc. 2016, 27(8), 1398-1405
Synthesis, in vitro Antiproliferative and Anti-Mycobacterium tuberculosis Activities of Novel β-Carboline Derivatives
Flora M. F. Moreira; Julio Croda; Maria H. Sarragiotto; Mary A. Foglio; Ana L. T. G. Ruiz; Joao E. Carvalho; Anelise S. N. Formagio
How to cite this article
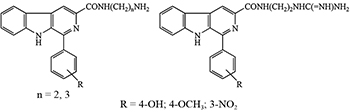
Novel 1-substituted-phenyl-β-carbolines with an amino or guanidinium group-terminated side chain at C-3 were evaluated for in vitro anti-tuberculosis, antiproliferative properties and through in silico study.
https://dx.doi.org/10.5935/0103-5053.20160062
J. Braz. Chem. Soc. 2016, 27(8), 1406-1412
Development of HPLC Analytical Techniques for Diterpene Glycosides from Stevia rebaudiana (Bertoni) Bertoni: Strategies to Scale-Up
Douglas L. Rodenburg; Kamilla Alves; Wilmer H. Perera; Taylor Ramsaroop; Raquel Carvalho; James D. McChesney
How to cite this article
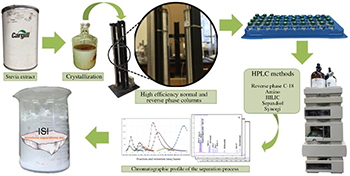
Large scale purification process for steviol glycosides from a commercial crude extract of Stevia rebaudiana.
https://dx.doi.org/10.5935/0103-5053.20160082
J. Braz. Chem. Soc. 2016, 27(8), 1413-1420
An Insight Into the Intraspecific Variation of Biosynthetic Gene Clusters Between Strains of Burkholderia thailandensis spp.
Joao Luiz Baldim; Marisi Gomes Soares
How to cite this article
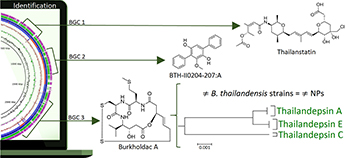
Replicons of Burkholderia thailandensis strains related to the production of three natural products (NPs). The biosynthetic gene clusters (BGCs) related to burkholdac were further analyzed and their side chain differentiation were tracked according to their genomes explaining the side chains modifications for this NP.
https://dx.doi.org/10.5935/0103-5053.20160108
J. Braz. Chem. Soc. 2016, 27(8), 1421-1431
New Dereplication Method Applied to NMR-Based Metabolomics on Different Fusarium Species Isolated from Rhizosphere of Senna spectabilis
Denise M. Selegato; Rafael T. Freire; Alberto Tannús; Ian Castro-Gamboa
How to cite this article
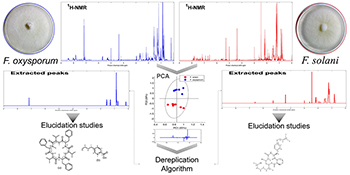
Workflow of the dereplication method: 1H NMR data from F. oxyporum and F. solani extracts were subjected to principal component analysis (PCA) for the selection of important chemical shifts region (loading values), followed by dereplication algorithm that extracts real 1H NMR peaks from the selected loading regions, enabling, through the comparison with an in-house NMR libraries and online databases, the identification of important bioactive metabolites in complex mixture.
https://dx.doi.org/10.5935/0103-5053.20160139
J. Braz. Chem. Soc. 2016, 27(8), 1432-1436
Annularins I and J: New Metabolites Isolated from Endophytic Fungus Exserohilum rostratum
Eduardo A. A. Pinheiro; Fabio C. Borges; Jeferson R. S. Pina; Leila R. S. Ferreira; Jorgeffson S. Cordeiro; Josiwander M. Carvalho; André O. Feitosa; Francinete R. Campos; Andersson Barison; Afonso D. L. Souza; Patrícia S. B. Marinho; Andrey M. R. Marinho
How to cite this article
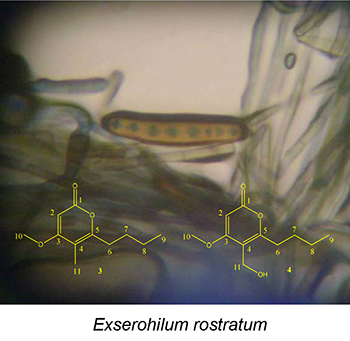
This paper describes the isolation of the compounds ergosterol peroxide, monocerin, annularin I and annularin J of the biomass extracts of Exserohilum rostratum. The compounds annularin I and annularin J are new natural products.
https://dx.doi.org/10.5935/0103-5053.20160140
J. Braz. Chem. Soc. 2016, 27(8), 1437-1443
Pyrrolizidine Alkaloids in the Pericopine Moth Scearctia figulina (Erebidae: Arctiinae): Metabolism and Chemical Defense
Carlos H. Z. Martins; José R. Trigo
How to cite this article
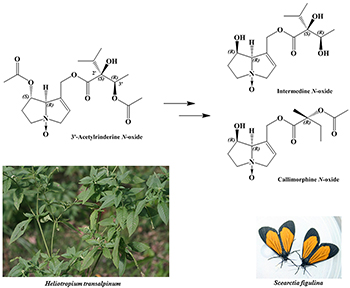
The pericopine moth Scearctia figulina transforms pyrrolizidine alkaloids from their host plant Heliotropium transalpinum.
https://dx.doi.org/10.5935/0103-5053.20160154
J. Braz. Chem. Soc. 2016, 27(8), 1444-1451
Copper and Manganese Cations Alter Secondary Metabolism in the Fungus Penicillium brasilianum
Taícia Pacheco Fill; Heloisa Fassina Pallini; Luciana da Silva Amaral; José Vinicius da Silva; Danielle Lazarin Bidóia; Francieli Peron; Francielle Pelegrin Garcia; Celso Vataru Nakamura; Edson Rodrigues-Filho
How to cite this article
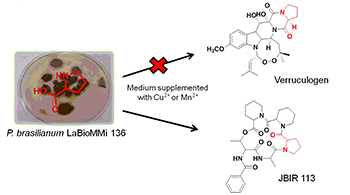
Medium composition modification locked verruculogen biosynthesis and addressed proline to the production of the cyclodepsipeptide JBIR 113 by the fungus P. brasilianum.
https://dx.doi.org/10.5935/0103-5053.20160163
J. Braz. Chem. Soc. 2016, 27(8), 1452-1458
Generation of Volatile Compounds from Carotenoids of Dunaliella bardawil Algae by Water Bath Heating and Microwave Irradiation
Natália A. B. Tinoco; Thais M. Uekane; Anna Tsukui; Paula F. de Aguiar; Cláudia M. L. L. Teixeira; Claudia M. Rezende
How to cite this article
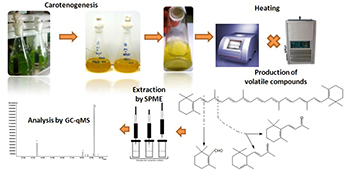
Volatiles from Dunaliella bardawil carotenoids produced by microwave irradiation and water bath controlled heating.
https://dx.doi.org/10.5935/0103-5053.20160170
J. Braz. Chem. Soc. 2016, 27(8), 1459-1464
(E)-4-Oxo-2-hexenal Dimers in the Scent Glands of the Bark Bug Phloea subquadrata (Heteroptera, Phloeidae)
Francine S. A. da Fonseca; Marília Medeiros; Adriana T. Salomao; Joao Vasconcellos-Neto; Anita J. Marsaioli
How to cite this article

Phloea subquadrata (Heteroptera, Phloeidae) and two molecules of (E)-4-oxo-2-hexenal which dimerize in the scent gland.
https://dx.doi.org/10.5935/0103-5053.20160179
J. Braz. Chem. Soc. 2016, 27(8), 1465-1475
Genome Mining of Endophytic Streptomyces wadayamensis Reveals High Antibiotic Production Capability
Célio F. F. Angolini; Ana B. Gonçalves; Renata Sigrist; Bruno S. Paulo; Markiyan Samborskyy; Pedro L. R. Cruz; Adriana F. Vivian; Eduardo M. Schmidt; Marcos N. Eberlin; Welington L. Araújo; Luciana G. de Oliveira
How to cite this article
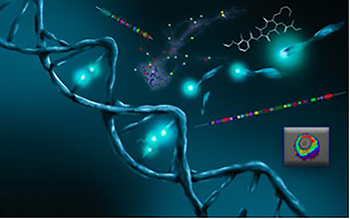
Mining DNA coupled with networking and analytical tools is a new-fashioned strategy to guide dereplication and finding for new therapeutic targets.
https://dx.doi.org/10.5935/0103-5053.20160180
J. Braz. Chem. Soc. 2016, 27(8), 1476-1483
Chemical Profiling of Ginseng Species and Ginseng Herbal Products Using UPLC/QTOF-MS
Jimmy Yuk; Dhavalkumar N. Patel; Giorgis Isaac; Kerri Smith; Mark Wrona; Hernando J. Olivos; Kate Yu
How to cite this article
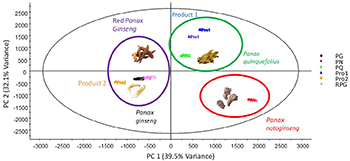
Classification of authentic and commercial ginseng using multivariate statistical analysis with ultra-performance liquid chromatography (UPLC)-quadrupole time-of-flight (QTOF)-mass spectrometry.
https://dx.doi.org/10.5935/0103-5053.20160189
J. Braz. Chem. Soc. 2016, 27(8), 1484-1492
Diurnal Pattern of Leaf, Flower and Fruit Specific Ambient Volatiles above an Oil Palm Plantation in Pará State, Brazil
Kolby J. Jardine; Bruno O. Gimenez; Alessandro C. Araújo; Roberto L. Cunha; Juliana Feitosa Felizzola; Luani R. Piva; Jeffrey Q. Chambers; Niro Higuchi
How to cite this article
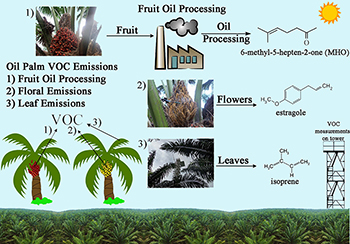
Tissue specific volatile organic compounds (VOC) emissions from Oil Palm plantations into the atmosphere during fruit oil processing resulting in emissions of 6-methyl-5-hepten-2-one (MHO) as an oxidation biomarker of lycopene; pollinator attraction resulting in floral emissions of estragole, and leaf photosynthesis resulting in leaf emissions of isoprene. Individual tissue measurements of VOC emissions were made using plant enclosure methods while diurnal concentrations of ambient VOCs were made on a walkup flux tower at the plantation scale near Belém, Brazil.
https://dx.doi.org/10.5935/0103-5053.20160194
J. Braz. Chem. Soc. 2016, 27(8), 1493-1505
Rapid Detection of ACTG- and AK-Toxins in Alternaria alternata by LC-ESI-MS/MS Analysis and Antifungal Properties of Citrus Compounds
Kátia R. Prieto; Lívia S. de Medeiros; Marsele M. Isidoro; Leonardo Toffano; Maria Fátima G. F. da Silva; Joao B. Fernandes; Paulo C. Vieira; Moacir R. Forim; Edson Rodrigues-Filho; Rodrigo M. Stuart; Marcos A. Machado
How to cite this article
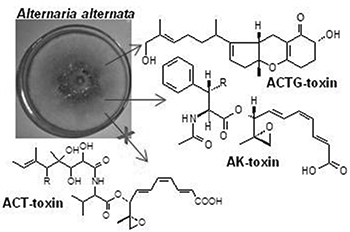
Using LC-ESI-MS/MS analysis, five toxins ACTG-C, D, E and F, and AK-toxin II were identified in A. alternata, ACT-toxins appear to be absent.
https://dx.doi.org/10.5935/0103-5053.20160195
J. Braz. Chem. Soc. 2016, 27(8), 1506-1511
Identification and Synthesis of the Male-Produced Sex Pheromone of the Soldier Beetle Chauliognathus fallax (Coleoptera: Cantharidae)
Diogo M. Vidal; Carla F. Fávaro; Matheus M. Guimaraes; Paulo H. G. Zarbin
How to cite this article
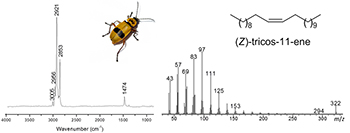
This paper describes the structure elucidation of the male-produced sex pheromone of the soldier beetle Chauliognathus fallax through GC-EAD, GC-MS and GC-FTIR analyses, microderivatization and synthesis. The natural compound was identified as (Z)-tricos-11-ene.
https://dx.doi.org/10.5935/0103-5053.20160199
J. Braz. Chem. Soc. 2016, 27(8), 1512-1519
Urease Inhibitors of Agricultural Interest Inspired by Structures of Plant Phenolic Aldehydes
Lívia P. Horta; Yane C. C. Mota; Gisele Maria Barbosa; Taniris C. Braga; Ivanildo E. Marriel; Angelo de Fátima; Luzia V. Modolo
How to cite this article
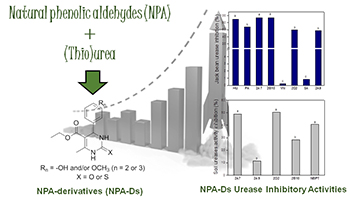
Hybrids of plant phenolic natural products protocatechuic aldehyde, syringaldehyde or vanillin with (thio)urea were obtained and investigated for the potential as urease inhibitors of agricultural interest.
https://dx.doi.org/10.21577/0103-5053.20160208
Online version ISSN 1678-4790 Printed version ISSN 0103-5053
Journal of the Brazilian Chemical Society
JBCS Editorial and Publishing Office
University of Campinas - UNICAMP
13083-970 Campinas-SP, Brazil
Free access










