vol. 27, No. 3, 2016, p. 435-639
Dihydrobetulinic acid (DHBA) is active against amastigotes and promastigotes from Leishmania donovani both in vitro and in vivo models. This compound inhibits the formation of the binary complex between topoisomerase IB (topIB) and DNA, and induces apoptosis. Based on docking and molecular dynamics studies we have identified the most probable binding site of DHBA on topIB. This computational model also revealed that a molecular fragment of this triterpenoid can be strategically relevant to develop new antileishmanial leads. Details are presented in the Article The Octahydroindene Carboxyl Substructure from Dihydrobetulinic Acid is Essential to Inhibit Topoisomerase IB from Leishmania donovani by Camila A. Rocha, Paulo R. S. Sanches, Reinaldo Marchetto and Aderson Zottis on page 591.
The Octahydroindene Carboxyl Substructure from Dihydrobetulinic Acid is Essential to Inhibit Topoisomerase IB from Leishmania donovani
Camila A. Rocha; Paulo R. S. Sanches; Reinaldo Marchetto; Aderson Zottis
How to cite this article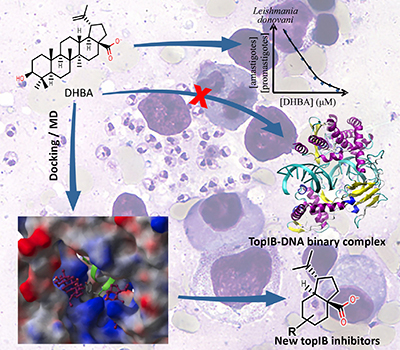
Dihydrobetulinic acid (DHBA) is active against amastigotes and promastigotes from Leishmania donovani both in vitro and in vivo models. This compound inhibits the formation of the binary complex between topoisomerase IB (topIB) and DNA, and induces apoptosis. Based on docking and molecular dynamics studies we have identified the most probable binding site of DHBA on topIB. This computational model also revealed that a molecular fragment of this triterpenoid can be strategically relevant to develop new antileishmanial leads. Details are presented in the Article The Octahydroindene Carboxyl Substructure from Dihydrobetulinic Acid is Essential to Inhibit Topoisomerase IB from Leishmania donovani by Camila A. Rocha, Paulo R. S. Sanches, Reinaldo Marchetto and Aderson Zottis on page 591.
https://dx.doi.org/10.5935/0103-5053.20150295
Review J. Braz. Chem. Soc. 2016, 27(3), 435-474
Antinociceptive Effect of Essential Oils and Their Constituents: an Update Review
Eder J. Lenardao; Lucielli Savegnago; Raquel G. Jacob; Francine N. Victoria; Débora M. Martinez
How to cite this article
Plant essential oils (EOs) have been used for centuries in folk medicine to treat diverse disorders, including as analgesic to pain relief. This review covers the literature on the antinociceptive activity of EOs and their constituents from 2000 to 2015. The concepts involved in the nociception, the major clinical treatments used to treat pain and the main tests used to access the analgesic effect of natural compounds are discussed.
https://dx.doi.org/10.5935/0103-5053.20150332
Articles J. Braz. Chem. Soc. 2016, 27(3), 475-483
Application of a Quantitative HPLC-ESI-MS/MS Method for Flavonoids in Different Vegetables Matrices
Bruno Perlatti; Joao B. Fernandes; Maria F. G. F. Silva; Jorge A. Ardila; Renato L. Carneiro; Bruno H. S. Souza; Eduardo N. Costa; Wellington I. Eduardo; Arlindo L. Boiça Junior; Moacir R. Forim
How to cite this article
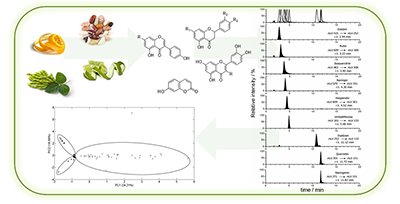
Different vegetable matrices were evaluated and discriminated by quantitative analysis of specific flavonoids using high-performance liquid chromatography coupled to electrospray ionization tandem mass spectrometry (HPLC-ESI-MS/MS) and principal component analysis (PCA).
https://dx.doi.org/10.5935/0103-5053.20150273
J. Braz. Chem. Soc. 2016, 27(3), 484-492
Synthesis of Organotin Substituted Tricyclic Macrodiolides
Flavia C. Zacconi; Romina A. Ocampo; Julio C. Podestá; Liliana C. Koll
How to cite this article
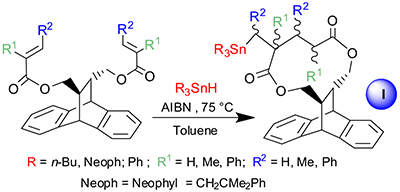
Synthesis of organotin substituted 11 membered macrocycles.
https://dx.doi.org/10.5935/0103-5053.20150275
J. Braz. Chem. Soc. 2016, 27(3), 493-499
Effect of Organic Solvent Composition on Dissociation Constants of Some Reversible Acetylcholinesterase Inhibitors
Y. Doğan Daldal; Ebru Çubuk Demiralay; Sibel A. Ozkan
How to cite this article
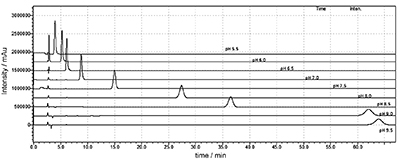
Variation of the mobile phase pH is a key parameter to enhance the chromatographic selectivity and retention time for ionization compounds. Solvent effect was investigated for the developed method. Dissociation constant values were calculated by different percentages of acetonitrile/methanol in the mobile phase at different pH environment. This work can be used for drug discovery of acetylcholinesterase inhibitors.
https://dx.doi.org/10.5935/0103-5053.20150276
J. Braz. Chem. Soc. 2016, 27(3), 500-509
Application of a New Strategy of Validation Based on "β,γ-Content Tolerance Interval" for Checking the Chiral Chromatography Method for Quantification of the Chiral Impurity of Levofloxacin
Houda Bouchafra; Miloud El karbane; Mohamed Azougagh; Fayssal Jhilal; Saad Alawi Sosse; EL Mestafa El hadrami; Taoufiq Saffaj; Bouchaîb Ihssane
How to cite this article
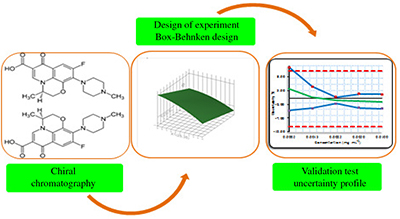
In order to get a chromatographic method able to yield a good separation, identification and quantification of the enantiomers (Levofloxacin and its (R)-enantiomer), chemometric tools should be appropriately applied, especially by applying the experimental designs methodology. In addition, in order to verify the optimized method we propose an innovative approach, recently developed in our laboratory called the uncertainty profile.
https://dx.doi.org/10.5935/0103-5053.20150277
J. Braz. Chem. Soc. 2016, 27(3), 510-514
Meroterpenoid Hydroquinones from Cordia globosa
Ana Karine O. Silva; André Luis L. de Oliveira; Francisco das Chagas L. Pinto; Karisia S. B. de Lima; Raimundo Braz-Filho; Edilberto R. Silveira; Otilia Deusdênia L. Pessoa
How to cite this article
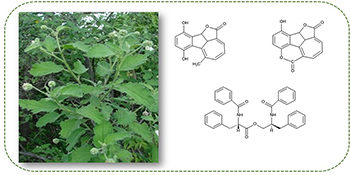
Two new meroterpenoid hydroquinones along with the known peptide (S)-N-benzoylphenylalanine-(S)-2-benzamide-3-phenylpropyl ester were isolated from the roots of Cordia globosa. Their structures were determined by 1D and 2D nuclear magnetic resonance (NMR) spectrometry, Fourier transform infrared (FTIR) spectroscopy and high resolution atmospheric pressure chemical ionization mass spectrometry (HRAPCIMS).
https://dx.doi.org/10.5935/0103-5053.20150278
J. Braz. Chem. Soc. 2016, 27(3), 515-525
Cr/Al Oxide as Solid Acid Catalyst to Afford Babassu Biodisel
Carla V. R. Moura; Haroldo L. S. Neres; Mariane G. Lima; Edmilson M. Moura; Jose M. Moita Neto; José E. de Oliveira; José R. O. Lima; Ilza M. Sttolin; Eugênio C. E. Araújo
How to cite this article
Solid acid catalyst was obtained by the mixture of Al/Cr oxides to afford babassu biodiesel with a great acidity 5.0%. The yield of biodiesel was about 97.5% in 15 h and 70 ºC.
https://dx.doi.org/10.5935/0103-5053.20150279
J. Braz. Chem. Soc. 2016, 27(3), 526-533
Alternative Igniters Based on Oxidant Salts for Microwave-Induced Combustion Method
Leticia S. F. Pereira; Gabrielle D. Iop; Mariele S. Nascimento; Liange O. Diehl; Cezar A. Bizzi; Juliano S. Barin; Erico M. M. Flores
How to cite this article
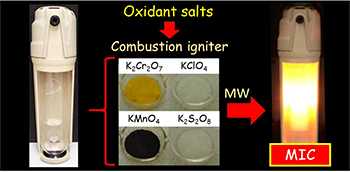
Use of solutions of oxidant salts as igniters, understanding the ignition assisted by microwaves, and the use of alternative igniters for microwave-induced combustion for further metals determination.
https://dx.doi.org/10.5935/0103-5053.20150280
J. Braz. Chem. Soc. 2016, 27(3), 535-545
Aminonaphthoquinone Mannich Bases Derived from Lawsone and Their Copper(II) Complex Derivatives: Synthesis and Potential Cholinesterase Inhibitors as Identified by On-flow Assay
Adriana F. L. Vilela; Bárbara M. Frugeri; André L. F. Sarria; Rodrigo O. S. Kitamura; Joao B. Fernandes; Maria F. G. F. Silva; Quezia B. Cass; Carmen L. Cardoso
How to cite this article
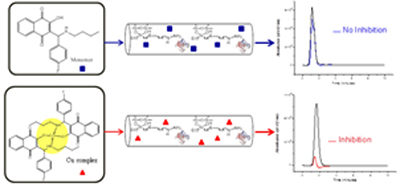
A new series of Mannich bases and their Cu2+ complexes were synthesized and evaluated for their potential as selective cholinesterase [acetylcholinesterase (AChE) and butyrylcholinesterase (BChE)] inhibitors by on-flow assay. All copper complexes were more active than the Mannich bases.
https://dx.doi.org/10.5935/0103-5053.20150281
J. Braz. Chem. Soc. 2016, 27(3), 546-550
A Greener, Efficient and Catalyst-Free Ultrasonic-Assisted Protocol for the N-Fmoc Protection of Amines
Rachida Mansouri; Zineb Aouf; Salah Lakrout; Malika Berredjem; Nour-Eddine Aouf
How to cite this article
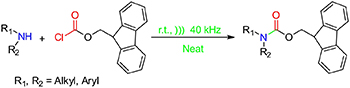
An eco-sustainable ultrasound-assisted method for the N-Fmoc protection is reported. Mildness, efficiency, excellent yielding and short reaction times are the main advantages of this new protocol.
https://dx.doi.org/10.5935/0103-5053.20150286
J. Braz. Chem. Soc. 2016, 27(3), 551-565
Synthesis, in vitro Antimalarial Activity and in silico Studies of Hybrid Kauranoid 1,2,3-Triazoles Derived from Naturally Occurring Diterpenes
Juliana de O. Santos; Guilherme R. Pereira; Geraldo C. Brandao; Tatiane F. Borgati; Lucas M. Arantes; Renata C. de Paula; Luciana F. Soares; Maria F. A. do Nascimento; Márlia R. C. Ferreira; Alex G. Taranto; Fernando P. Varotti; Alaíde B. de Oliveira
How to cite this article
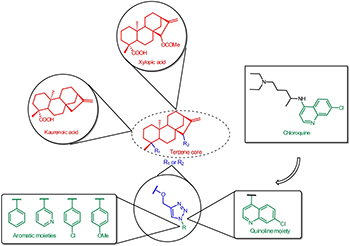
The kauranoid moieties core in the novel 1,2,3-triazole-1,4-disubstituted hybrid molecules were synthesized by copper-catalyzed azide-alkyne cycloaddition (CuAAC) reactions. All compounds were evaluated for antiplasmodial and cytotoxic activity.
https://dx.doi.org/10.5935/0103-5053.20150287
J. Braz. Chem. Soc. 2016, 27(3), 566-574
Determination of Aspirin Using Chemiluminescence System of Tris(1,10 phenanthroline)Ruthenium(II)-Cerium(IV)
Ali Mokhtari; Mohsen Keyvanfard; Iraj Emami; Nastaran J. Delouei; Hatameh F. Pishkhani; Aida Ebrahimi; Hossein Karimian
How to cite this article
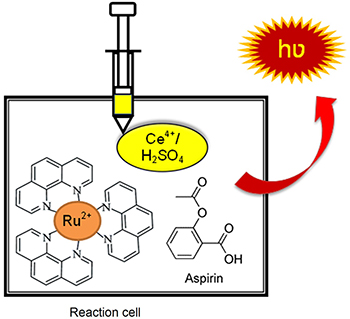
A chemiluminescence method based on the enhancement effect of aspirin in the system of tris(1,10 phenanthroline)ruthenium(II), with acidic CeIV has been proposed.
https://dx.doi.org/10.5935/0103-5053.20150288
J. Braz. Chem. Soc. 2016, 27(3), 575-583
Use of Natural Rubber Membranes as Support for Powder TiO2 and Ag/TiO2 Photocatalysts
Jusinei M. Stropa; Aline S. Herrero; Silvio C. Oliveira; Alberto A. Cavalheiro; Renato F. Dantas; Samuel L. Oliveira; Amilcar Machulek Jr; Lincoln C. S. Oliveira
How to cite this article
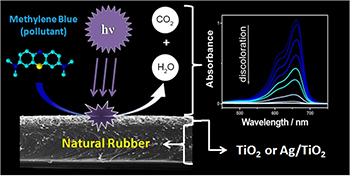
Methylene blue discoloration on a natural rubber membrane surface containing TiO2 or Ag/TiO2. On ultraviolet-visible (UV-Vis) spectrophotometric analysis, decreased absorbance intensity indicates methylene blue degradation.
https://dx.doi.org/10.5935/0103-5053.20150293
J. Braz. Chem. Soc. 2016, 27(3), 584-590
Pressurized Flow Solubilization System Using Electromagnetic Induction Heating Technique for Simultaneous Determination of Inorganic Elements (Ba, Ca, Cd, Cu, Fe, Mg, Mn, Na, Pb, Sr, Zn) in Sonicate Slurries of Biological Materials by Microwave Induced Plasma Optical Emission Spectrometry (MIP-OES)
Henryk Matusiewicz; Mariusz Ślachciński
How to cite this article
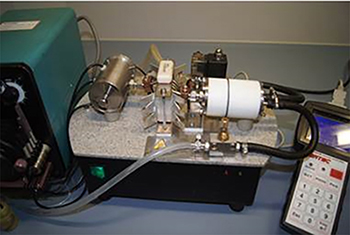
Pressure electromagnetic induction heating flow solubilization system.
https://dx.doi.org/10.5935/0103-5053.20150294
J. Braz. Chem. Soc. 2016, 27(3), 591-598
The Octahydroindene Carboxyl Substructure from Dihydrobetulinic Acid is Essential to Inhibit Topoisomerase IB from Leishmania donovani
Camila A. Rocha; Paulo R. S. Sanches; Reinaldo Marchetto; Aderson Zottis
How to cite this article
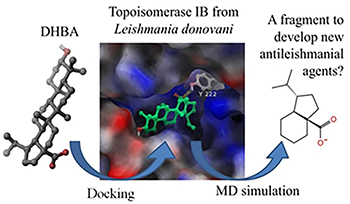
Dihydrobetulinic acid is a competitive inhibitor of topoisomerase IB from Leishmania donovani. By docking and simulations of molecular dynamics it was identified a potential fragment to develop new antileishmanial compounds.
https://dx.doi.org/10.5935/0103-5053.20150295
J. Braz. Chem. Soc. 2016, 27(3), 599-604
Chemotaxonomy of the Amazonian Unonopsis Species Based on Leaf Alkaloid Fingerprint Direct Infusion ESI-MS and Chemometric Analysis
Felipe M. A. Silva; Francinaldo A. Silva Filho; Bruna R. Lima; Richardson A. Almeida; Elzalina R. Soares; Hector H. F. Koolen; Afonso D. L. Souza; Maria L. B. Pinheiro
How to cite this article
Alkaloid fingerprint along with multivariate analysis provided a simple and effective approach to differentiate Amazonian Unonopsis species.
https://dx.doi.org/10.5935/0103-5053.20150296
J. Braz. Chem. Soc. 2016, 27(3), 605-615
AC Induced Corrosion of Underground Steel Pipelines. Faradaic Rectification under Cathodic Protection: II. Theoretical Approach with Electrolyte Resistance and Double Layer Capacitance for Bi-Tafelian Corrosion Mechanism
Ibrahim Ibrahim; Michel Meyer; Hisasi Takenouti; Bernard Tribollet
How to cite this article
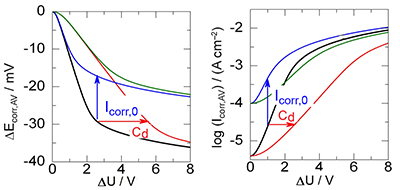
Effect of the AC stray voltage on the faradaic rectification by the double layer capacitance and the corrosion current density.
https://dx.doi.org/10.5935/0103-5053.20150302
J. Braz. Chem. Soc. 2016, 27(3), 616-623
Characterization of Pequi (Caryocar brasiliense) Shells and Evaluation of Their Potential for the Adsorption of PbII Ions in Aqueous Systems
Dayane J. Amorim; Hélen C. Rezende; Érica L. Oliveira; Ione L. S. Almeida; Nívia M. M. Coelho; Túlio N. Matos; Cleide S. T. Araújo
How to cite this article
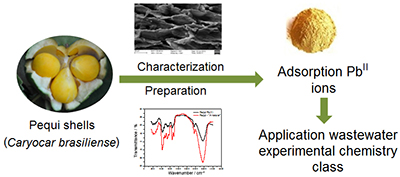
Pequi (Caryocar brasiliense) shells were characterized by point of zero charge (PZC), Fourier transform infrared spectroscopy (FTIR), and scanning electron microscopy-energy dispersive spectroscopy (SEM-EDS) and the adsorbent material was studied for PbII ions adsorption.
https://dx.doi.org/10.5935/0103-5053.20150304
J. Braz. Chem. Soc. 2016, 27(3), 624-630
Determination of Amphetamine, Amfepramone and Fenproporex in Urine Samples by HPLC-DAD: Application to a Population of Brazilian Truck Drivers
Juliana Takitane; Rafael M. Almeida; Tiago F. Oliveira; Natanael V. Prado; Daniel R. Muñoz; Vilma Leyton; Mauricio Yonamine
How to cite this article
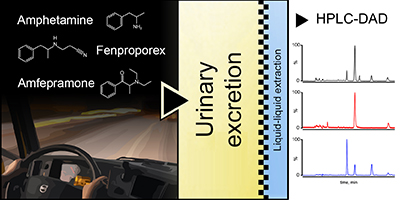
Liquid-liquid extraction was applied in urine samples collected from truck drivers for the determination of amphetamines by high performance liquid chromatography with diode array detection (HPLC-DAD).
https://dx.doi.org/10.5935/0103-5053.20150305
Short Report J. Braz. Chem. Soc. 2016, 27(3), 631-638
Effect of Polyethyleneglycols (PEG) on Solubility of CoIII 5,10,15,20-Tetra(4-carboxyphenyl)porphyrin and Methylimidazolyl Axial Complex at 298.2 K: Experiment and Modeling
Tatyana V. Volkova; Vasiliy A. Golubev; Mikhail Y. Nikiforov; Galina M. Mamardashvili; Nugzar Z. Mamardashvili; German L. Perlovich
How to cite this article
In this study simple and efficient method of enhancing the solubility of CoIII porphyrins based on polyethyleneglycol additions to water solution was proposed.
https://dx.doi.org/10.5935/0103-5053.20150307
Additions and Corrections J. Braz. Chem. Soc. 2016, 27(3), 639
Equilibrium and Out-Of-Equilibrium Investigation of Proton Exchange and CuII and ZnII Complexation on Fungal Mycelium (Trametes hirsuta)
Vicente R. Almeida; Bruno Szpoganicz; Lei Chou; Kitty Baert; Annick Hubin; Steeve Bonneville
How to cite this article
https://dx.doi.org/10.5935/0103-5053.20160025
Online version ISSN 1678-4790 Printed version ISSN 0103-5053
Journal of the Brazilian Chemical Society
JBCS Editorial and Publishing Office
University of Campinas - UNICAMP
13083-970 Campinas-SP, Brazil
Free access










