vol. 30, No. 1, 2019, p. 1-209
Several studies have reported the anticarcinogenic activity of conjugated linoleic acid (CLA) naturally found in the meat of ruminants (cattle, goats, and sheep) and in dairy products. The anticarcinogenic effect of the cis-9, trans-11 isomer of CLA was studied using two breast cancer (BC) cell lines, one positive (MCF-7) and the other negative (MDA-MB-231) for estrogen receptors. The nuclear magnetic resonance (NMR) metabolomics studies of intact BC cells demonstrate that CLA reduces the level of phosphocholine, a BC malignancy biomarker, in both cell lines. CLA also strongly interferes in acetone metabolism, mainly in the MCF-7 cell line, indicating an effect on fatty acid metabolism. Details are presented in the Communication Effect of cis-9, trans-11 Conjugated Linoleic Acid (CLA) on the Metabolism Profile of Breast Cancer Cells Determined by 1H HR-MAS NMR Spectroscopy by Roberta M. Maria, Wanessa F. Altei, Napoleao F. Valadares, Richard C. Garratt, Adriano D. Andricopulo, Tiago Venâncio and Luiz A. Colnago on page 3.
Effect of cis-9, trans-11 Conjugated Linoleic Acid (CLA) on the Metabolism Profile of Breast Cancer Cells Determined by 1H HR-MAS NMR Spectroscopy
Roberta M. Maria  ; Wanessa F. Altei; Napoleao F. Valadares; Richard C. Garratt; Adriano D. Andricopulo; Tiago Venâncio; Luiz A. Colnago
; Wanessa F. Altei; Napoleao F. Valadares; Richard C. Garratt; Adriano D. Andricopulo; Tiago Venâncio; Luiz A. Colnago
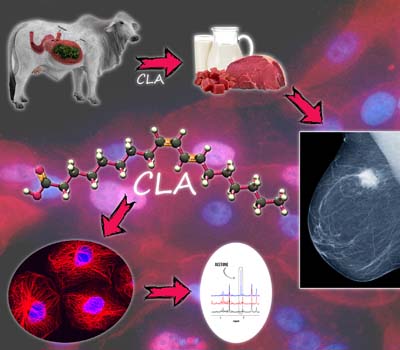
Several studies have reported the anticarcinogenic activity of conjugated linoleic acid (CLA) naturally found in the meat of ruminants (cattle, goats, and sheep) and in dairy products. The anticarcinogenic effect of the cis-9, trans-11 isomer of CLA was studied using two breast cancer (BC) cell lines, one positive (MCF-7) and the other negative (MDA-MB-231) for estrogen receptors. The nuclear magnetic resonance (NMR) metabolomics studies of intact BC cells demonstrate that CLA reduces the level of phosphocholine, a BC malignancy biomarker, in both cell lines. CLA also strongly interferes in acetone metabolism, mainly in the MCF-7 cell line, indicating an effect on fatty acid metabolism. Details are presented in the Communication Effect of cis-9, trans-11 Conjugated Linoleic Acid (CLA) on the Metabolism Profile of Breast Cancer Cells Determined by 1H HR-MAS NMR Spectroscopy by Roberta M. Maria, Wanessa F. Altei, Napoleao F. Valadares, Richard C. Garratt, Adriano D. Andricopulo, Tiago Venâncio and Luiz A. Colnago on page 3.
https://dx.doi.org/10.21577/0103-5053.20180200
Editorial J. Braz. Chem. Soc. 2019, 30(1), 1-2
Communication J. Braz. Chem. Soc. 2019, 30(1), 3-7
Effect of cis-9, trans-11 Conjugated Linoleic Acid (CLA) on the Metabolism Profile of Breast Cancer Cells Determined by 1H HR-MAS NMR Spectroscopy
Roberta M. Maria  ; Wanessa F. Altei; Napoleao F. Valadares; Richard C. Garratt; Adriano D. Andricopulo; Tiago Venâncio; Luiz A. Colnago
; Wanessa F. Altei; Napoleao F. Valadares; Richard C. Garratt; Adriano D. Andricopulo; Tiago Venâncio; Luiz A. Colnago
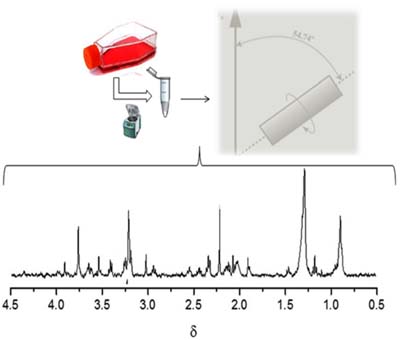
The effects of cis-9, trans-11 conjugated linoleic acid (CLA) on the metabolic profile of MCF-7 and MDA-MB-231 breast cancer cells were evaluated using high-resolution magic angle spinning (HR-MAS) nuclear magnetic resonance (NMR) spectroscopy.
https://dx.doi.org/10.21577/0103-5053.20180200
Articles J. Braz. Chem. Soc. 2019, 30(1), 8-18
Synthesis and Cytotoxicity Evaluation of a Series of 3-Alkenyl-2-Hydroxy-1,4‑Naphthoquinones Obtained by an Efficient Knoevenagel Condensation
Cibelle C. David; Antonio C. S. Lins; Tania M. S. Silva; Júlia F. Campos; Teresinha G. Silva; Gardenia C. G. Militao; Celso A. Camara
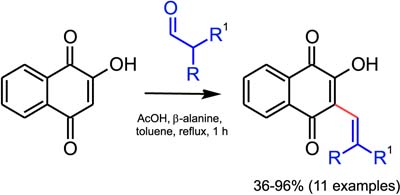
An efficient Knoevenagel condensation between aldehydes and lawsone was performed under new conditions showing good isolated yields in a short reaction time and simple conditions.
https://dx.doi.org/10.21577/0103-5053.20180146
J. Braz. Chem. Soc. 2019, 30(1), 19-32
Synthesis of Spiro-Pyrrolidinyloxindoles by Oxidative Rearrangement of N-Acyltetrahydro-β-carbolines Using an Oxone/Aqueous Acetone Mixture
Luisa L. Marçal; Simon J. Garden
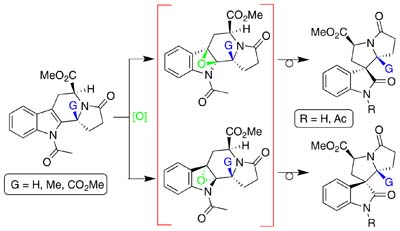
Substrate face controlled concerted oxidative rearrangement of N-acyltetrahydro-β-carbolines stereoselectively gives spiropyrrolidinyloxindoles.
https://dx.doi.org/10.21577/0103-5053.20180149
J. Braz. Chem. Soc. 2019, 30(1), 33-47
Pyrazolyl-Tetrazoles and Imidazolyl-Pyrazoles as Potential Anticoagulants and their Integrated Multiplex Analysis Virtual Screening
André L. P. G. Lourenço; Percilene F. Vegi; Jéssica V. Faria; Gustavo S. P. Pinto; Maurício S. dos Santos  ; Plínio C. Sathler; Max S. Saito; Marcos Santana; Tatiana P. P. Dutra; Carlos R. Rodrigues; Robson Q. Monteiro; Alice M. R. Bernardino; Helena C. Castro
; Plínio C. Sathler; Max S. Saito; Marcos Santana; Tatiana P. P. Dutra; Carlos R. Rodrigues; Robson Q. Monteiro; Alice M. R. Bernardino; Helena C. Castro
Fourteen pyrazole compounds were synthesized and evaluated with regard to anticoagulant and antithrombotic activities. These derivatives were investigated through an integrated multiplex analysis virtual screening (IMA-VS) pipeline.
https://dx.doi.org/10.21577/0103-5053.20180150
J. Braz. Chem. Soc. 2019, 30(1), 48-59
Determination of Parabens in Breast Milk Samples by Dispersive Liquid-Liquid Microextraction (DLLME) and Ultra-High-Performance Liquid Chromatography Tandem Mass Spectrometry
Caroline F. Grecco; Israel D. Souza; Vinicius R. Acquaro Junior; Maria E. C. Queiroz
Determination of parabens in breast milk samples by dispersive liquid-liquid microextraction (DLLME) and ultra-high-performance liquid chromatography tandem mass spectrometry (UHPLC-MS/MS).
https://dx.doi.org/10.21577/0103-5053.20180151
J. Braz. Chem. Soc. 2019, 30(1), 60-70
Analysis of Isomeric Cannabinoid Standards and Cannabis Products by UPLC-ESI-TWIM-MS: a Comparison with GC-MS and GC x GC-QMS
Nayara A. dos Santos; Lilian V. Tose; Samantha R. C. da Silva; Michael Murgu; Ricardo M. Kuster; Rafael S. Ortiz; Flavio A. O. Camargo; Boniek G. Vaz; Valdemar Lacerda Jr.  ; Wanderson Romao
; Wanderson Romao
Analysis of cannabinoid standards by ultra performance liquid chromatography coupled with electrospray ionization-quadrupole-time of flight-mass spectrometry (UPLC-ESI-QTOF-MS) and UPLC-ESI-travelling wave ion mobility (TWIM)-MS.
https://dx.doi.org/10.21577/0103-5053.20180152
J. Braz. Chem. Soc. 2019, 30(1), 71-80
Occurrence of Pesticides and PPCPs in Surface and Drinking Water in Southern Brazil: Data on 4-Year Monitoring
Sergiane S. Caldas; Jean Lucas O. Arias  ; Caroline Rombaldi; Lucas L. Mello; Maristela B. R. Cerqueira; Ayrton F. Martins; Ednei G. Primel
; Caroline Rombaldi; Lucas L. Mello; Maristela B. R. Cerqueira; Ayrton F. Martins; Ednei G. Primel
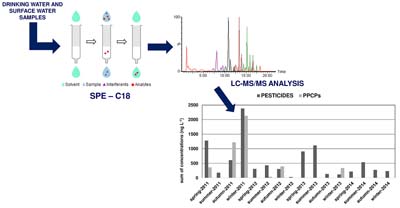
Fifty-one compounds including PPCPs and pesticides were monitored from 2011 to 2014 in drinking and surface waters in Rio Grande do Sul State.
https://dx.doi.org/10.21577/0103-5053.20180154
J. Braz. Chem. Soc. 2019, 30(1), 81-89
Synthesis, Photophysical and Electrochemical Properties of Novel D-π-D and D-π-A Triphenylamino-Chalcones and β-Arylchalcones
Rafaela G. M. da Costa; Francisco R. L. Farias; Luis Maqueira; Carlos Castanho Neto; Leonardo S. A. Carneiro; Joseany M. S. Almeida; Camilla D. Buarque; Ricardo Q. Aucélio; Jones Limberger
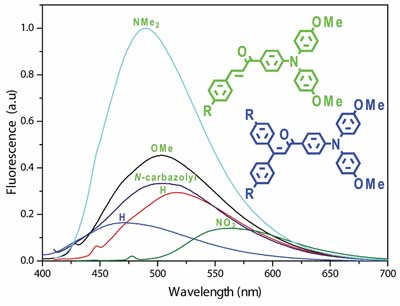
A series of triphenylamino (TPA)-chalcones and β-arylchalcones, displaying either D-π-D or D-π-A architecture, were synthesized and characterized. Photophysical and electrochemical properties of these compounds were evaluated.
https://dx.doi.org/10.21577/0103-5053.20180156
J. Braz. Chem. Soc. 2019, 30(1), 90-96
Influence of Copper and Metallic Alloys on the Oxidation Reaction of Commercial Biodiesel in Mixture with Natural Antioxidant
Letícia T. Chendynski; Érica S. Romagnoli; Ana Carolina G. Mantovani; Marissa Kimura; Leonardo C. Marques; Dionisio Borsato
Influence of copper and metallic alloys on the biodiesel oxidation reaction.
https://dx.doi.org/10.21577/0103-5053.20180157
J. Braz. Chem. Soc. 2019, 30(1), 97-107
Synthesis of 1,2,3-Triazole Derivatives of 4,4'-Dihydroxybenzophenone and Evaluation of Their Elastase Inhibitory Activity
Maria C. F. Dias; Thiago Q. Gularte; Róbson R. Teixeira  ; Jorge A. N. Santos; Eduardo J. Pilau
; Jorge A. N. Santos; Eduardo J. Pilau  ; Tiago A. O. Mendes; Antônio J. Demuner; Marcelo H. dos Santos
; Tiago A. O. Mendes; Antônio J. Demuner; Marcelo H. dos Santos
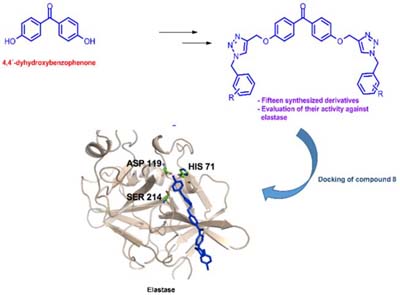
The conversion of 4,4'-dihydroxybenzophenone into a series of 1,2,3-triazolic derivatives resulted in the identification of bis(4-(1-(4-iodobenzyl)-1H-1,2,3-triazol-4-yl)methoxy)benzophenone with significant elastase inhibitory activity (IC50 = 16.6 ± 1.9 µM, inhibition constant (Ki) of 11.12 µM).
https://dx.doi.org/10.21577/0103-5053.20180158
J. Braz. Chem. Soc. 2019, 30(1), 108-115
In vitro Evaluation of Ca, Cu, and Mg Bioaccessibility in Fresh and Dried Fruits
Thais T. Mingroni; Juliana Hamada; Alexsandra D. S. Xavier; Cleusa Cavalcante; Angerson N. do Nascimento
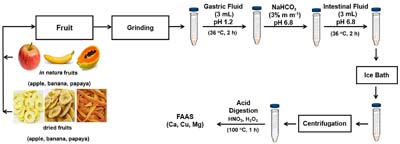
An in vitro evaluation of Ca, Cu, and Mg bioaccessibility in fresh and dried fruits was carried out, with results demonstrating that the dehydration process negatively affects bioaccessibility of Ca and Mg in dried fruits.
https://dx.doi.org/10.21577/0103-5053.20180159
J. Braz. Chem. Soc. 2019, 30(1), 116-123
Anti-Parasite Activity of Novel 3,5-Diiodophenethyl-benzamides
Manuel Pastrana Restrepo  ; Verónica Surmay Surmay; Elkin Galeano Jaramillo; Sara Robledo Restrepo
; Verónica Surmay Surmay; Elkin Galeano Jaramillo; Sara Robledo Restrepo
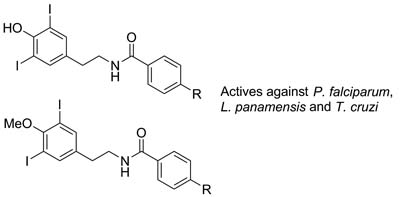
Novel 3,5-diiodophenethyl-benzamides as new lead compounds for the development of new anti-parasites treatment.
https://dx.doi.org/10.21577/0103-5053.20180160
J. Braz. Chem. Soc. 2019, 30(1), 124-131
Eco-Friendly Synthesis of 2,3-Dihydroquinazolin-4(1H)-ones Catalyzed by FeCl3/Al2O3 and Analysis of Large 1H NMR Diastereotopic Effect
Isabel Monreal; Mariano Sánchez-Castellanos; Karla Ramírez‑Gualito; Gabriel Cuevas; Karla A. Espinoza; Ignacio A. Rivero
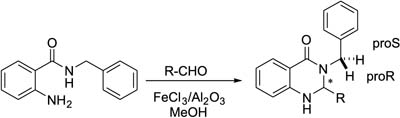
The quinazolinones showed a large range diastereotopic effect that was studied through nuclear magnetic resonance (NMR) and computational calculations.
https://dx.doi.org/10.21577/0103-5053.20180161
J. Braz. Chem. Soc. 2019, 30(1), 132-139
Ultrasonic Extracts of Morinda citrifolia L.: Characterization of Volatile Compounds by Gas Chromatography-Mass Spectrometry
Daiane B. M. Lima; Anaí L. dos Santos; Ariel O. Celestino; Nayna Sampaio; Jéssica Baldez; Maria I. S. Melecchi; Thiago R. Bjerk; Laíza C. Krause; Elina B. Caramao
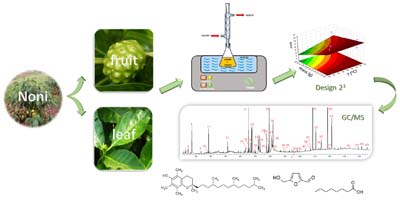
Sonication extraction from leaves and fruits using response surface methodology and analysis of the extracts by gas chromatography-mass spectrometry (GC-MS) showing as major compounds: octanoic acid, vitamin E, phytol and hydroxymethylfurfural.
https://dx.doi.org/10.21577/0103-5053.20180162
J. Braz. Chem. Soc. 2019, 30(1), 140-148
Sample Preparation Applied to Analysis of Proteins in Cowpea Seeds (Vigna unguiculata (L.) Walp.)
Thábata L. A. B. Nascimento; Tiago L. S. Coelho; Cícero A. Lopes Júnior; Samuel A. A. Sousa; Herbert S. Barbosa
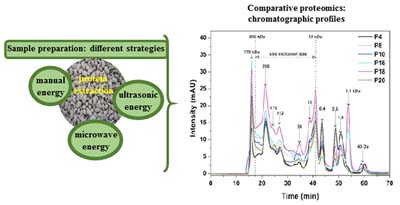
Optimization of sample preparation procedure in the extraction of cowpea proteins using the high-performance liquid chromatography (HPLC) technique as a comparative tool.
https://dx.doi.org/10.21577/0103-5053.20180163
J. Braz. Chem. Soc. 2019, 30(1), 149-157
Biotechnological Properties of Sponges from Northeast Brazil: Cliona varians as a Biocatalyst for Enantioselective Reduction of Carbonyl Compounds
Valéria B. Riatto  ; Mauricio M. Victor; Jaqueline F. Sousa; Carla Menegola
; Mauricio M. Victor; Jaqueline F. Sousa; Carla Menegola

This work describes the first use of marine sponge Cliona varians as biocatalytic agents in the stereoselective reduction of α-keto esters and isatin.
https://dx.doi.org/10.21577/0103-5053.20180165
J. Braz. Chem. Soc. 2019, 30(1), 158-163
Male-Specific Volatiles Released by the Big Avocado Seed Weevil Heilipus lauri Boheman (Coleoptera: Curculionidae)
Alicia A. Romero-Frías  ; Diana C. Sinuco; José Maurício S. Bento
; Diana C. Sinuco; José Maurício S. Bento
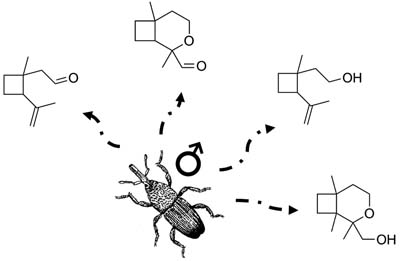
Volatile organic compounds released by Heilipus lauri (Coleoptera: Curculionidae).
https://dx.doi.org/10.21577/0103-5053.20180166
J. Braz. Chem. Soc. 2019, 30(1), 164-172
Synthesis, Antimicrobial Activity and Structure-Activity Relationship of Some 5-Arylidene-thiazolidine-2,4-dione Derivatives
Raíssa K. C. de Paiva; Jamerson F. da Silva; Hudieyllen A. Moreira; Osvaldo G. Pinto; Lilian T. F. M. Camargo; Plínio L. F. Naves; Ademir J. Camargo; Luciano Ribeiro; Luciana M. Ramos
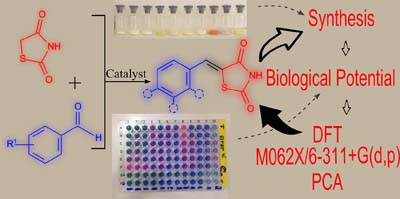
The synthesis and antimicrobial activity of thiazolidine-2,4-dione derivatives are described. The structures of the synthesized compounds were confirmed by infrared and nuclear magnetic resonance. All compounds were evaluated for their in vitro antibacterial activity. Density functional theory and principal component analysis were used to discriminate the compounds.
https://dx.doi.org/10.21577/0103-5053.20180167
J. Braz. Chem. Soc. 2019, 30(1), 173-179
On the Use of an Interpolation Approach for the Choice of Gaussian Polarization Functions
Fernando Ratuchne; Ana C. Mora; Ricardo Celeste; Albérico B. F. da Silva
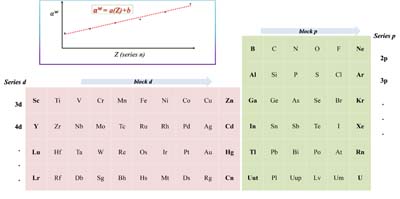
The polarization exponents (αw) vary with the atomic number (Z) for a sequence of atoms in a row with the same valence structure. Thus, αw can be obtained by interpolation.
https://dx.doi.org/10.21577/0103-5053.20180168
J. Braz. Chem. Soc. 2019, 30(1), 180-187
Use of Coconut Charcoal and Menthone-Thiosemicarbazone Polymer as Solid Phase Materials for the Determination of N,N-Dimethyltryptamine, Harmine, Harmaline, Harmalol, and Tetrahydroharmine in Ayahuasca Beverage by Liquid Chromatography-Tandem Mass Spectrometry
Sandro Navickiene  ; Luis F. S. Santos; Mônica C. Santos; Alain Gaujac
; Luis F. S. Santos; Mônica C. Santos; Alain Gaujac
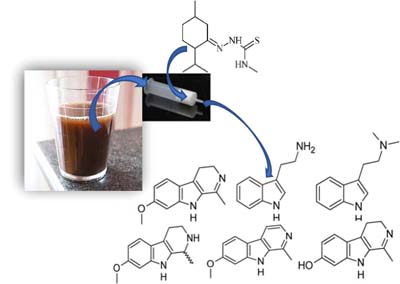
Evaluation of efficiency of coconut charcoal and menthone-thiosemicarbazone as alternative solid phase extraction adsorbent for the determination of alkaloids in ayahuasca beverage.
https://dx.doi.org/10.21577/0103-5053.20180169
Short Report J. Braz. Chem. Soc. 2019, 30(1), 188-197
Synthesis, in silico Study and Antimicrobial Evaluation of New Selenoglycolicamides
Helivaldo D. S. Souza; Roxana P. F. de Sousa; Bruno F. Lira; Raquel F. Vilela; Nathalie H. P. B. Borges; José P. de Siqueira‑Junior; Edeltrudes O. Lima; Jeane U. G. Jardim; Gracielle A. T. da Silva; José M. Barbosa‑Filho; Petrônio F. de Athayde‑Filho
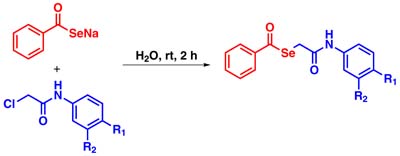
The organoselenium compounds presented are prominent in medicinal chemistry; due to the increase of resistant fungi and bacteria, novel organoselenium compounds have been investigated as potent antimicrobials.
https://dx.doi.org/10.21577/0103-5053.20180148
J. Braz. Chem. Soc. 2019, 30(1), 198-209
A Facile Synthesis of Novel Isatinspirooxazine Derivatives and Potential in vitro Anti-Proliferative Activity
Iara S. Santos; Fabiana S. Guerra; Lucas F. Bernardino; Patrícia D. Fernandes; Lidilhone Hamerski; Bárbara V. Silva
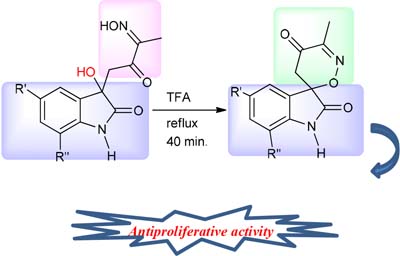
It was synthesized a new isatinspirooxazine derivative with an half maximal inhibitory concentration (IC50) = 0.34 µM, more potent than the reference drug, doxorubicin (IC50 = 1.88 µM), in breast cancer line MDA-MB231.
https://dx.doi.org/10.21577/0103-5053.20180153
Online version ISSN 1678-4790 Printed version ISSN 0103-5053
Journal of the Brazilian Chemical Society
JBCS Editorial and Publishing Office
University of Campinas - UNICAMP
13083-970 Campinas-SP, Brazil
Free access










